Multiple sclerosis and the experimental treatment giving families hope
MULTIPLE SCLEROSIS: THE BASICS
Multiple sclerosis is an autoimmune disease that can affect the brain and spinal cord.
It impacts 2.5 million people worldwide and it is estimated that 130,000 people in the UK have the condition. This means that in the UK one in 500 people has MS.
It's most commonly diagnosed in people in their 20s and 30s, although it can develop at any age.
You have probably heard of MS - but what actually happens to the body to cause these symptoms?
This video explains the science behind multiple sclerosis and the key to unlocking potential treatments:
Symptoms differ from person to person, but these are the most common:

While there are drugs available to slow the onset of symptoms, treatment options for progressive MS are limited and there is currently no cure. This is a scary fact for those living with the condition and for those 7,000 people being diagnosed every year in the UK.
A huge amount of money is being pumped into research. The MS Society has invested over £227 million into research of stem cells, immunology, symptom relief and MS services since 1956.
It hopes to raise another £100 million over ten years with its Stop MS appeal, launched in 2019, to dramatically advance research.
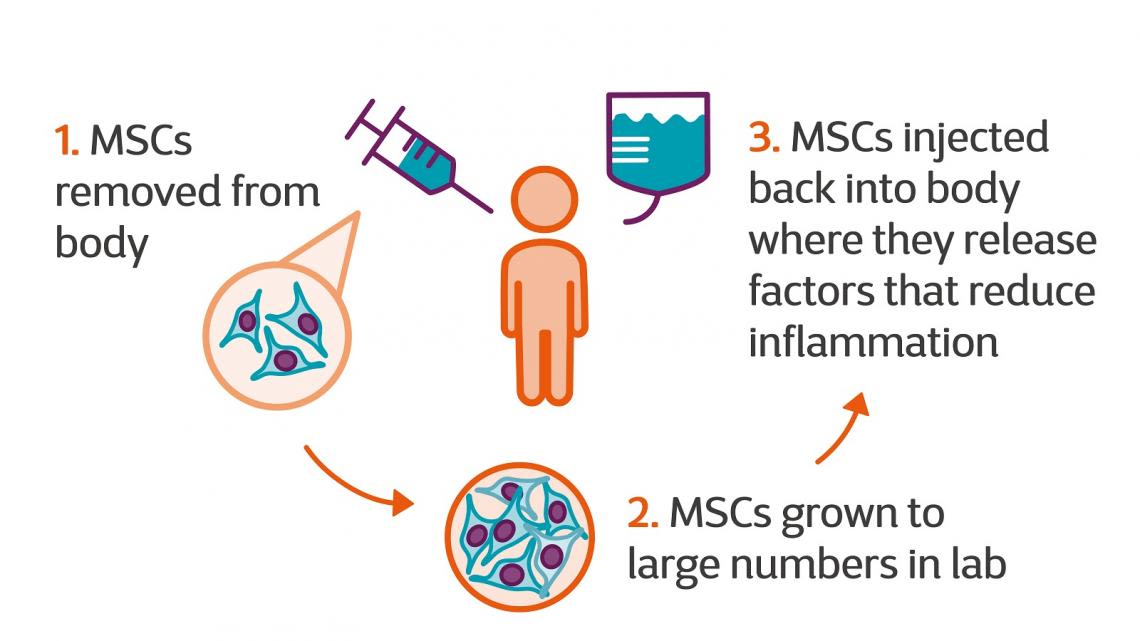
At the end of July, the US Food and Drug Administration authorised a Phase 2 clinical trial at Hope Biosciences Stem Cell Research Foundation in Texas. This trial will assess how effective infusions of mesenchymal stem cells are in improving MS symptoms and quality of life.
The future of MS treatment is exciting; scientists think solutions for all types of MS could be in their late stage trials by 2025.
But what are the options for those living with progressive MS right now? Some are desperate to halt their symptom deterioration and even reverse the damage already done.
Increasingly, they are looking to experimental treatments like the one offered by Panama’s Stem Cell Institute.

The Stem Cell Institute
This treatment centre in Panama administers mesenchymal stem cells which have been taken from donated umbilical cords and grown in labs.
Imperial College London tested MSCs from the patient's own bone marrow in 2019 and Hope Biosciences's trial in Texas will test them from fat tissue.
MSCs are adult cells which are present in different parts of the body.
MSCs can produce other cells like muscle. This is why researchers have been interested in testing whether they can protect nerves from more damage and repair the original damage.
The Stem Cell Institute's website says: "The organization arose from the unmet need to provide stem cell therapies that have been shown by others to meet the bar of safety in Phase I trials, but are not yet widely available because efficacy has not been proven."
Potential patients have to sign a disclaimer to show that they understand the treatments are not US FDA approved and that there could be unknown risks as long-term studies have not yet been performed.
Treatments at the Institute cost upwards of £17,000.
Like others with progressive MS, Alister Bailey and Liam Egalton see this as their only hope.
Both men have embarked on this treatment journey to do what they can to halt and reverse the effects of their condition.
Background: MSCs under a microscope
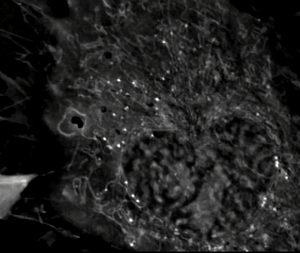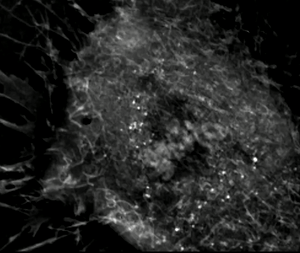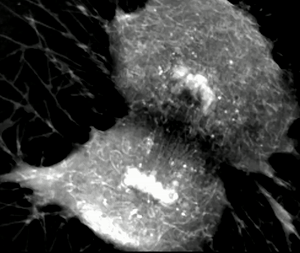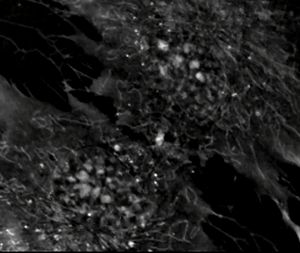
ALISTER'S STORY
“There’s no cure for progressive MS so this treatment in Panama is his only hope."
As a teenager, Alister, from Barnes, was into motorbike racing and ended up competing at British Championship Level. He appeared in a fly-on-the-wall TV series Natural Born Racers.

A year after he married Gemma, Alister started experiencing episodes where he wasn’t able to walk. But when he went blind for two days in 2012, he finally received his progressive MS diagnosis.
He has been on a drug for ten years which has slowed symptom progression but Gemma says his condition has rapidly deteriorated in the past three years. While he does not use a wheelchair at home, he cannot walk any long distance unsupported.
She said: “First of all, Alister didn’t qualify for anything on the NHS which was pretty disheartening.
“We have various doctor friends, some of them researching stem cell treatments for different illnesses but they said this is where medicine is going in the future.
“There are no negatives to it other than that it may not work.”
After raising £22,500, the pair were supposed to fly out to Panama for the first round of treatment in January. Coronavirus put a stop to those plans.
Travel restrictions meant they had to delay the trip until the end of April - but, even then, it was certainly not an easy ride. They were warned they still wouldn't make it to Panama with the travel restrictions.
Read below about their rollercoaster journey:

Since they returned, Alister has already started to see some benefits. Before Panama, he couldn’t hold a cup of tea, but now his hands have stopped shaking and he can pour a drink without spilling it. He has also noticed an improvement in his eyesight.
Gemma said: “This might seem trivial but it’s a huge physical change. That’s a really positive sign and certainly encouraging for us in terms of the next round.
"He really believes it will work and he feels something is happening."
What's next?
The Baileys are hoping to see the benefits of treatment after two or three rounds and have prepared themselves for potentially five visits.
They have planned fundraising events to get together the funds needed so they can return to Panama in November.
On 17 July, Alister's 67-year-old dad Chris took on the Surrey Hills Epic Off-Road Challenge which involves 125km of off-road mountain biking.
Gemma is organising a charity dinner and her friend Claire is running the Lulworth Cove Trail Challenge which involves running along the coastline.
Their friend Jonny Wright has already raised $14,400 as he prepares to cycle 2,500 miles from Maine to Florida in 30 days starting on 27 August.
Last week, Gemma took their son Ronnie on holiday. She went alone due to concerns that Alister wouldn't be able to get around or sleep properly.
Their hope is that the treatment will stimulate repair of the damaged tissues so that Alister can play football with his son again.
Check out Alister's Instagram page to keep up with their journey:
LIAM'S STORY
“With the rate of my decline, I haven’t got time to wait five or ten years to get on a trial."
Liam, a chartered surveyor from Essex, has secondary progressive MS. He flew out to Panama on Friday with his girlfriend for his fourth round of treatment. He’s been looking forward to this moment for over a year.
The journey has been turbulent, like Alister’s, and made even more unbearable by the pandemic.
Travel restrictions stopped him from returning to Panama earlier, and it has been 18 months since his last infusion of stem cells. Specialists at the Stem Cell Institute recommend six month gaps.
He has noticed a physical deterioration over the pandemic months.
“It’s been like hell the last year because you just see the decline in your health."
“I was nowhere near as poorly as I am now before coronavirus started.
“I live in a close and there’s a guy opposite me who has got really bad MS. I don’t want to get there. There’s nothing in the UK so that’s it now.
“I can’t describe how good it is to be going back. My body is gagging for it and I can’t wait.”
Liam has nothing but praise for the Stem Cell Institute which his personal trainer recommended.
“Until I found Panama, I thought I’m dead in the water here. There was nothing for me.”
He first noticed MS symptoms back in 2013 when he ran a marathon in seven and a half hours, three hours longer than he anticipated. He knew something wasn’t right when his calves cramped up.
In February of the following year, aged 30, he woke up and couldn’t see. When he tried to jump out of bed, his legs buckled below him. The next week was spent in hospital recovering.
He then raised over £60,000 for the first two trips.
Liam has seen benefits from each round of the treatment and is hoping for a complete reversal of the damage done to his body.
What benefits has Liam noticed from the first three rounds of treatment?
Are mesenchymal stem cells the future for MS treatment?
A look at the Texas trial
On 27 July, the FDA gave the go-ahead for a Phase 2 clinical trial of mesenchymal stem cell treatment for MS.
The Hope Biosciences Stem Cell Research Foundation in Texas will test how effective multiple infusions of mesenchymal stem cells are in improving the symptoms of patients with mild-to-moderate MS.
Twenty-four patients will receive six infusions over 32 weeks.
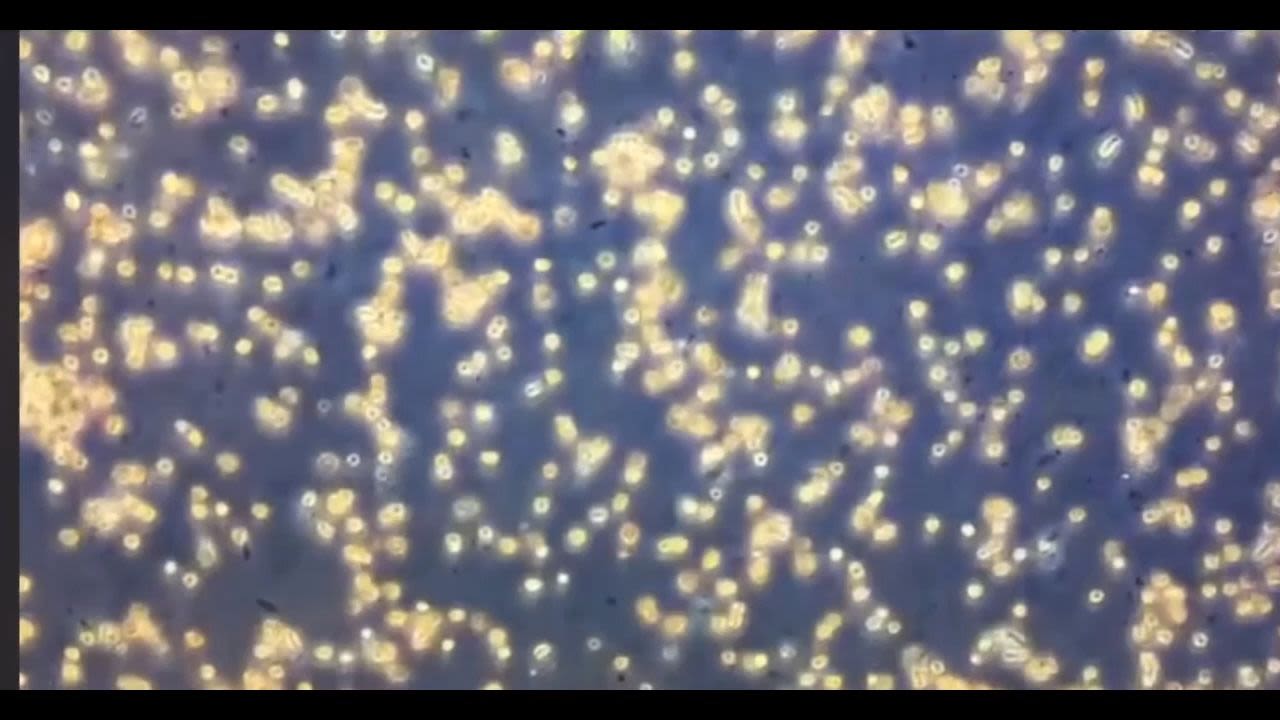
A fat sample will be isolated and cultured from the patient's own tissue.
Over the course of the study, patients will receive over 1.2 billion stem cells.
Jan Shultis, growth lead at Hope Biosciences said: "We are very hopeful and looking forward to results.
"Numbers are simply unheard of before because no-one had the technological problems solved to the extent that such a number of high quality cells could be produced in that volume.
"We will see what happens when you give more infusions, closer together for a longer period of time.
"The ultimate goal and vision is to make these treatments available to everyone, to really develop treatments for conditions that are right now considered incurable."
The team hopes to have a scientifically published result within a year.
MULTIPLE SCLEROSIS IN THE UK
The UK's MS Society has funded projects to improve diagnosis, treatments and services since 1956.
On the subject of mesenchymal stem cell therapy, senior research manager David Coutts said: "I recognise that there are some heartbreaking situations for people with MS and desperate choices that people are making. It is up to everyone to decide.
"For us, because we are an evidence-based organisation, we can only make recommendations based on what we think is the qualified evidence.
"We haven’t recommended MSCT treatment because there isn’t the clinical evidence yet to suggest it is of benefit, but that may change in the future. In the UK, it is still an experimental treatment in clinical trials."
What research projects are the MS Society funding now?
David Coutts, senior research manager at the MS Society, speaks about ongoing projects
David Coutts, senior research manager at the MS Society, speaks about ongoing projects
Coutts is hopeful about the development of new MS treatments.
"The best predictor of the future is what's happened in the past," he says.
"The rate of new treatment development in MS has been amongst the best for any neurological condition.
"In 25 years there has been over a dozen new treatments made available. That hopefully means a similar rate of progress can be made over the next five, ten years.
"I am genuinely really hopeful that in the lifespan of our Stop MS appeal that we will be able to see treatments in trials that benefit everyone with MS."
You can keep up to date with the MS Society's Stop MS appeal by following the hashtag #TeamStopMS on Twitter.

IV drip photo credit: pxfuel
MS symptoms diagram: created on Canva
What is MS? video credit: MS Society's YouTube
Scientist looking through microscope image credit: pxfuel
Mesenchymal stem cell gif credit: Evilonan via Creative Commons
Timeline infographic created on Flourish. Credit for all images: Gemma Bailey
Stem cells video credit: Hope Biosciences
Nurse with IV bag photo credit: Hope Biosciences Stem Cell Research Foundation
Patients receiving treatment photo credit: Hope Biosciences Stem Cell Research
Scientist in lab photo credit: Pixabay
